15. While mercury is very useful in barometers, mercury vapor is toxic. Given that mercury has a  of 59.11 kJ/mol and its normal boiling point is 356.7℃, calculate the vapor pressure in mm Hg at room temperature, 25℃. (A)2.68 x 10-3 mm Hg (B) 2.99 mm Hg (C) 372 mm Hg (D) 753 mm Hg
of 59.11 kJ/mol and its normal boiling point is 356.7℃, calculate the vapor pressure in mm Hg at room temperature, 25℃. (A)2.68 x 10-3 mm Hg (B) 2.99 mm Hg (C) 372 mm Hg (D) 753 mm Hg
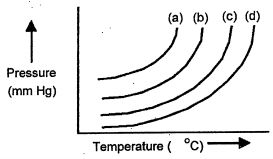
The plots below represent vapor pres assure vs, temperature curves for diethyl ether, ethanol, mercury, and water, not necessarily in that order.