21. For the reaction CH3CHCH2(g) + HCl(g) → CH3CHC1CH3(g)
a possible mechanism is
2 HCl ⇌(HCl)2 (rate constant: k₁ and k-1, fast equilibrium)
CH3CHCH2 + HCl ⇌Complex (rate constant: k2 and 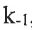 , fast equilibrium)
, fast equilibrium)
Complex + (HCl)2 →CH3CHCICH3 + 2HCl (rate constant k3, slow)
What's the rate law for this reaction? (A)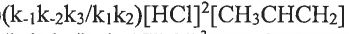 (B)
(B)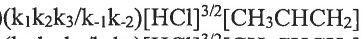 (C)
(C) (D)
(D)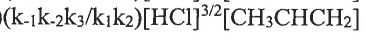 (E)
(E)