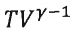23.Based on the first law of thermodynamics, in adiabatic ideal-gas processes, which one should be a nonzero constant? Note that ΔEint, Q, W, p, V, γ, and T are the change in the system's internal energy, the heat supplied to the system, the work done on the system, the gas pressure, the gas volume, the ratio of the specific heats at constant pressure and at constant volume (=  ), and the gas temperature, respectively. (A)
), and the gas temperature, respectively. (A) (B) Q (C) W (D)
(B) Q (C) W (D)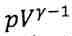 (E)
(E)