19. In an organic solution, an organometallic compound R3MPH3 (R = alkyl, M = metal) decomposes into a hydrocarbon R-R and a new compound RMPH3. The mechanism for the reaction was proposed as follows. The first and third steps are fast, whereas the second step is slow. What is the rate law based on the mechanism?
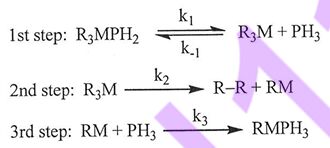
(A) Rate =(k1k3/k-1)R3MPH3[PH3]
(B) Rate = R-R/[R3M]
(C) Rate = (k2k1/k-1)[R3MPH3]/[PH3]
(D) Rate = k2[R3M]/[PH3]
(E) Rate = k1[R3MPH3]
答案:登入後查看
統計: A(0), B(1), C(1), D(1), E(0) #3422490
統計: A(0), B(1), C(1), D(1), E(0) #3422490