90. ff a 20.0 g sample of CaCO3 is put into a 20.0 L container and heated to 800℃
, what the mass percentage of the CaCO3 will react to reach equilibrium? (Ca=40.08 g/mol), 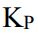 = 1.16 at 800℃
= 1.16 at 800℃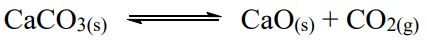
(A) 100%
(B) 76%
(C) 50%
(D) 24%
(E) 13%
答案:登入後查看
統計: A(2), B(0), C(2), D(0), E(1) #3429609
統計: A(2), B(0), C(2), D(0), E(1) #3429609