題組內容
1. Methanol is synthesized by the reaction of carbon monoxide with hydrogen,
CO + 2H2→CH3OH
15% of CO is converted to CH3OH (the density is 74 g/L) in the reactor. From the reactor, the material flows to a separator where the pure CH3OH product is separated as a liquid from the unreacted gases. These gases are then recycled back to the feed stream so that the process runs economically. The process is shown below, 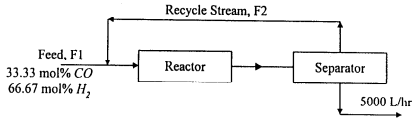

Calculate