題組內容
8.We consider the irreversible freezing of water below its freezing point. The freezing of a mole of water at -10°C is an irreversible change, but can be carried out reversibly by means of the three steps.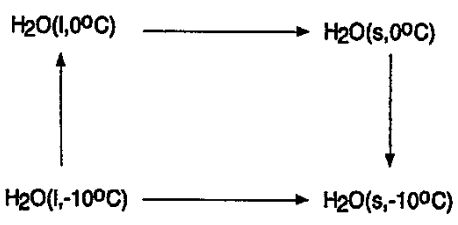
(b) (5%) The entropy of change for the isolated system upon freezing includes the entropy change of the surrounding as the entropy change of the water. Since the heat capacity of surrounding is large, the heat evolved by the water on freezing us absorbed by the surrounding with only an infinitesimal change in temperature. Calculate the entropy change of the surrounding! What is the change in the entropy of the universe?