題組內容
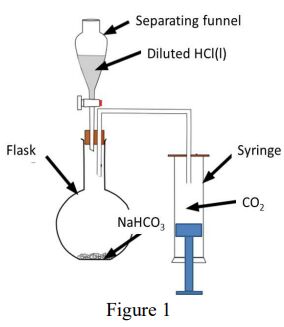
30. The questions from 30a to 30c share the same conditions. In the experiment of determining molecular mass of carbon dioxide, CO2, the ideal gas equation, PV = nRT, is employed to calculate the molecular mass, g mol−1 . The equipment of preparation of CO2 is shown as figure 1. Table 1 shows the data of the experiment results, the temperature, the pressure, the mass of the syringe with the rubber bung, and the mass of the syringe with the bung containing carbon dioxide. The volume of the syringe is 60.0 cm3
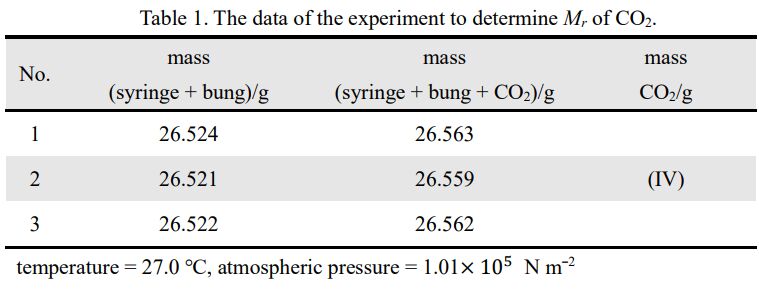
30c. The molecular mass determined by the ideal gas equation is quite different from the molecular mass of CO2,
44 g mol−1
. Explain the reason that causes the difference. [1 marks]