題組內容
3. Enzyme E catalyzes the unimolecular reaction S→P following Michaelis-Menton kinetics such that the reaction rate v follows 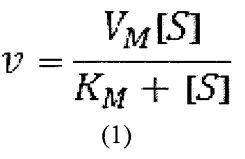
We learn that inhibitor I can inhibit the reaction such that the inhibited kinetics follow the Lineweaver-Burk plot below
What kind of inhibition is this? Competitive, noncompetitive or uncompetitive (1%); Does the inhibitor compete the same binding site with the substrate or not? (1%) We chemically modify I into I'; now I' binds the enzyme even tighter than before. How are the lines in the figure going to shift? Compare [I'] with [I] at the same concentrations (2%). Describe two examples how enzymes regulate their activities in vivo? (2%)