題組內容
11.At high temperature, a system at 1 bar is in gas phase with three components: A, B, and C in a molar
ratio of 2:1:2. The temperature goes down at a fixed pressure of 1 bar, and we observe that the bubble point
occurs at 49 'C. The vapor pressures of the three species are the following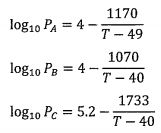
where the pressure is in a unit of bar and temperature is in a unit of K.