30. Shown below is a phase diagram for sulfur (not drawn to scale). Sulfur can exist in solid modifications, rhombic and monoclinic, denoted by  , respectively. Which of the following statements is incorrect?
, respectively. Which of the following statements is incorrect?
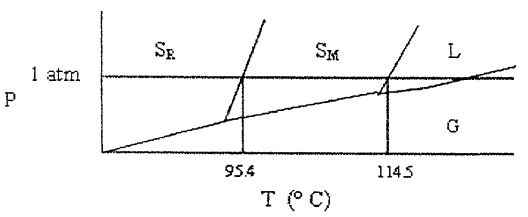 (A) At pressures close to 1 atm, rhombic sulfur can be in stable equilibrium with liquid sulfur. (B) Under ordinary atmospheric conditions (at sea level), sulfur does not sublime. (C) This system has two triple points. (D) At a given pressure, there is (at most) one temperature at which rhombic sulfur can exist in equilibrium with monoclinic sulfur. (E) None of these statements is incorrect.
(A) At pressures close to 1 atm, rhombic sulfur can be in stable equilibrium with liquid sulfur. (B) Under ordinary atmospheric conditions (at sea level), sulfur does not sublime. (C) This system has two triple points. (D) At a given pressure, there is (at most) one temperature at which rhombic sulfur can exist in equilibrium with monoclinic sulfur. (E) None of these statements is incorrect.