The * symbol in the first reaction indicates the reactant is activated through collision. Experimental ly, it is observed that the reaction can be either first or second order under different conditions. The rate law is expressed as
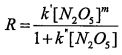
. Which of the following statement is true? (A)m = 2. (B) k'=k
1 (C)

(D)When

, the reaction will be first order in N
2O
5(g). (E) When

, the reaction will be half order in N
2O
5(g).