複選題
2. (20 points total) Calculate the pH when 100.0 mL of a 1.00 M solution of H2A (
 ) is titrated with the following volumes of 1.00 M NaOH.
) is titrated with the following volumes of 1.00 M NaOH.
(A) (5 points) Calculate the initial pH of the solution.
(B) (5 points) Calculate the pH of the resulting solution after 75.0 mL of 1.00 M NaOH has been added.
(C) (5 points) Calculate the pH of the resulting solution after 100.0 mL of 1.00 M NaOH has been added.
(D) (5 points) Calculate the 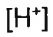 of the resulting solution after 200.0 mL of 1.00 M NaOH has been added.
of the resulting solution after 200.0 mL of 1.00 M NaOH has been added.
答案:登入後查看
統計: 尚無統計資料
統計: 尚無統計資料