26. Redox chemistry
(26A, 5%) Balance the following redox reaction under acidic conditions and indicate the oxidizing agent and the reducing agent:  (1)
(1)
(26B, 2%) Write down the expression of the equilibrium constant K. for reaction (1) after balancing.
(26C, 3%) The reaction (1) after balancing has a standard electromotive force (E0) of 0.56V. Calculate the value of the equilibrium constant K. at 25°C.
 Planck constant h = 6.62608 x
Planck constant h = 6.62608 x  J s
J s
Rydberg constant RH = 2.180 ×  J
J
Avogadro constant Na = 6.02214 × 
Faraday constant F = 96485 C 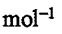
Ideal gas constant R = 0.08206 L atm  = 62.36 L torr
= 62.36 L torr  = 8.314 J
= 8.314 J 
△G° = ΔΗ° - TASO
△G° = -RTln K = -nFE° (cell)
Energy levels of hydrogenic atom: 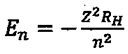
Boiling-point elevation: 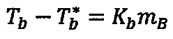
Freezing-point depression: 
First-order reaction: [A] = 
Second-order reaction: 1/[A] = 1/[A]0 + kt
Arrhenius equation: k = 