13. One ionic crystal structure contains two ions, 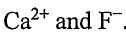 .
.  ions occupy the face-centered cubic lattice points, and
ions occupy the face-centered cubic lattice points, and 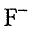 ions occupy all the tetrahedral holes. Which statement is invalid?
ions occupy all the tetrahedral holes. Which statement is invalid?
(A) The ionic radius of
of 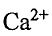 is larger than the ionic radius
is larger than the ionic radius 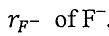 .
.
(B) The coordination numbers of 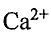 and F- are 12 and 8 respectively.
and F- are 12 and 8 respectively.
(C) The relation between the edge length a of the unit cell and  is equivalent to a * √3/4 =
is equivalent to a * √3/4 =  .
.
(D) There are 4 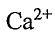 ions and 8 F- ions in a unit cell.
ions and 8 F- ions in a unit cell.
(E) The empirical formula of the ionic crystal structure is CaF2.
答案:登入後查看
統計: 尚無統計資料
統計: 尚無統計資料