2. Given the 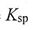 values for the following compounds:
values for the following compounds:

Which of the following statements about these compounds is incorrect ?
(A) The solubility of MnS in water is pH dependent.
(B) Zn(OH)2 has the lowest molar solubility in water.
(C) Pb(OH)2 has the highest molar solubility in water.
(D) Zn(OH)2 is more soluble upon decreasing the pH of solution.
(E) A saturated PbCrO4 solution has a lower  than a saturated Pb(OH)2 solution.
than a saturated Pb(OH)2 solution.
答案:登入後查看
統計: A(0), B(1), C(1), D(0), E(1) #3422473
統計: A(0), B(1), C(1), D(0), E(1) #3422473