4. Consider the reaction: a X(g) = b Y(g)
Each entry in the table below represents the equilibrium partial pressures of X and Y under different initial conditions at the same temperature. The coefficients a and b are the simplest integers. When the equilibrium partial pressure of X is 0.20 atm, the equilibrium partial pressure of Y is n atm. Which of the following statements is true?
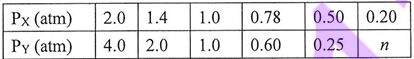
(A) a = 1
(B) b = 2
(C) n = 0.10
(D) a = 2b
(E) a + b = 4
答案:登入後查看
統計: A(0), B(1), C(0), D(2), E(0) #3422475
統計: A(0), B(1), C(0), D(2), E(0) #3422475