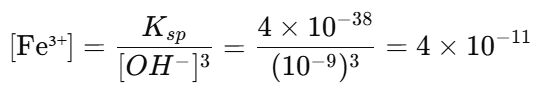20. The solubility of Fe(OH)3 (Ksp = 4 × 10-38) in a solution buffered at pH = 5.0 is mol/L
(A) 4.0 × 10-11
(B)2.5 × 10-10
(C) 2.5 × 10-4
(D) 4.0 × 10-29
(E)4.0 × 10-17
答案:登入後查看
統計: A(7), B(0), C(0), D(3), E(0) #2344112
統計: A(7), B(0), C(0), D(3), E(0) #2344112