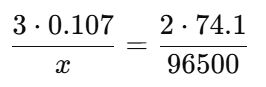23. An unknown metal M is electrolyzed. It took 74.1 s for a current of 2.00 A to plate out 0.107 g of the metal from a solution containing M(NO3)3. The metal is therefore identified as________ .
(A) aluminum
(B)cobalt
(C) bismuth
(D) lead
(E)gallium
答案:登入後查看
統計: A(2), B(4), C(3), D(0), E(0) #2344115
統計: A(2), B(4), C(3), D(0), E(0) #2344115