27. The formation of stalactite is closely related to the concentration of carbon- containing species formed by the dissolution of CaCO3 in the rocks and CO2 in the air in natural water bodies. Given that 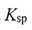 (CaCO3) =
(CaCO3) = 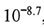 , pM = -log[M] where M =
, pM = -log[M] where M = 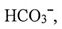
 , the variation of pM with pH in a cave water body at room temperature is shown on the right. Which of the following is incorrect ?
, the variation of pM with pH in a cave water body at room temperature is shown on the right. Which of the following is incorrect ? 
(A) represents  .
.
(B)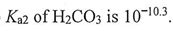
(C) m = 2.75 and n = 4.35
(D) When pH is 10.3, 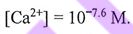
(E) When pH is increased from 4 to 8, 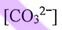 increases faster than
increases faster than  .
.
答案:登入後查看
統計: A(0), B(0), C(1), D(1), E(1) #3422498
統計: A(0), B(0), C(1), D(1), E(1) #3422498