28. At a certain temperature, cis-1,2-dimethylcyclopropane (M) and trans-1,2- dimethylcyclopropane (N) undergo the following transformation. 
The rate laws for the reactions can be expressed as 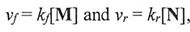 , with ky and k, being constants at a given temperature, called the forward and reverse rate constants, respectively, and the equilibrium constant of the reaction, K = 3. Which of the following statements is correct?
, with ky and k, being constants at a given temperature, called the forward and reverse rate constants, respectively, and the equilibrium constant of the reaction, K = 3. Which of the following statements is correct?
(A) At this temperature, the conversion of M is 66.7%.
(B) As the temperature increases, both 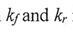 , increase.
, increase.
(C) At room temperature, N is a gas under 1 atm.
(D) M is more stable than N.
(E) Heating this reaction increases the equilibrium constant K.
答案:登入後查看
統計: A(0), B(2), C(0), D(0), E(1) #3422499
統計: A(0), B(2), C(0), D(0), E(1) #3422499