34. In glycolysis, fructose 1,6-bisphosphate is converted to two products with a standard free-energy change  of 23.8 kJ/mol. Under what conditions (encountered in a normal cell) will the free-energy change (AG) be negative, enabling the reaction to proceed to the right?
of 23.8 kJ/mol. Under what conditions (encountered in a normal cell) will the free-energy change (AG) be negative, enabling the reaction to proceed to the right?
(A) Under standard conditions, enough energy is released to drive the reaction to the right.
(B) If the concentrations of the two products are high relative to that of fructose 1,6-bisphosphate.
(C) The reaction will not go to the right spontaneously under any conditions because the 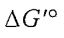 is positive.
is positive.
(D) When there is a high concentration of fructose 1,6-bisphosphate relative to the concentration of products.
(E) None of the above.
答案:登入後查看
統計: 尚無統計資料
統計: 尚無統計資料