40. 紫外光-可見光分光光譜(UV-Vis Spectroscopy)可利用 Beer’s Law 做濃度定量分析。 若 1.3 × 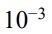 M 濃度之 KMnO4 樣品,其在波長為 555 nm 測得吸收值(Peak absorbance) 為 6.5,假設光程長度(Path length)為 1.0 cm,請問莫耳吸收係數(Molar absorptivity, ε )為何?
M 濃度之 KMnO4 樣品,其在波長為 555 nm 測得吸收值(Peak absorbance) 為 6.5,假設光程長度(Path length)為 1.0 cm,請問莫耳吸收係數(Molar absorptivity, ε )為何?
(A)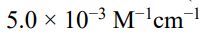
(B)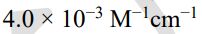
(C)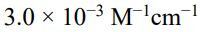
(D) 
答案:登入後查看
統計: A(22), B(6), C(8), D(4), E(0) #3430590
統計: A(22), B(6), C(8), D(4), E(0) #3430590
詳解 (共 6 筆)
#6406994

2
0
#7026285
21. Which one of the following descriptions for the Beer’s law is incorrect?
(A) Beer’s law is ordinarily represented as A =εbs.
(B) For a mixture, the total absorbance at a λ=the sum of individual absorbance if there is no intermolecular interaction.
(C) Beer’s law is more suitable for concentrated solutions.
(D) Negative deviations are always observed if polychromic radiations are used.
(E) The b term in the equation of item (a) means the optical length.
答案:C
ㅤㅤ
23. What is the Beer-Lambert law limitation for high concentrations?
(A) Absorbance decreases linearly.
(B) Stray light causes deviations.
(C) Band broadening occurs.
(D) Nonlinear absorbance-concentration relationship.
(E) Path length variations amplify errors.
答案:D
ㅤㅤ
30. In UV-Vis spectroscopy, the absorbance of light is proportional to:
(A) Wavelength.
(B) Concentration and path length.
(C) Temperature.
(D) Molecular weight.
(E) Molar extinction coefficient.
答案:B
ㅤㅤ
19. 下圖是吸光度(absorbance)對Co(II)濃度(mg/mL)的標準校準曲線 (standard calibration curve)。取 0.50 mL 未知濃度的 Co(II)溶液,並稀釋至10.0 mL。測試其吸光度為0.564。此未知溶液中Co(II)離子的濃度是多少?

(A) 0.017 mg/mL
(B) 0.17 mg/mL
(C) 0.34 mg/mL
(D) 0.56 mg/mL
答案:C
0
1