6. A chemist needs to prepare a solution buffered at pH 4.30 using the following acids and its sodium salt. Consider the ratio of [HA]/[A-] and pKa of each acid, which of the following acid can make the buffer system work best?
(A) Chloroacetic acid 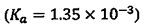
(B) Propanoic acid 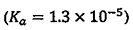
(C) Benzoic acid 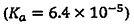
(D) Hypochlorous acid 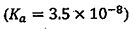
答案:登入後查看
統計: A(0), B(0), C(0), D(1), E(0) #3423509
統計: A(0), B(0), C(0), D(1), E(0) #3423509