68. Which of the following statements is incorrect?
(A) An orbital can accommodate at most two electrons with the same spin quantum number.
(B) The electron density at a point is disproportional to Ψ2 at that point.
(C) The  quantum number of an electron must be either +
quantum number of an electron must be either + 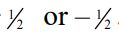 .
.
(D) A 2p orbital is more penetrating than a 2s; i.e., it has a higher electron density near the
nucleus and inside the charge cloud of a 1s orbital.
(E) All of these are incorrect.
答案:登入後查看
統計: A(0), B(0), C(2), D(1), E(3) #3429587
統計: A(0), B(0), C(2), D(1), E(3) #3429587