69. The solubility of the ionic compound M2X3, having a molar mass of 288 g/mol, is 3.60× g/Lat 25oC. What is the Ksp of the compound at 25℃?
g/Lat 25oC. What is the Ksp of the compound at 25℃?
(A)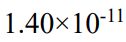
(B)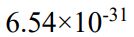
(C) 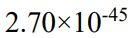
(D)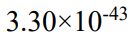
(E) 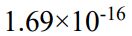
答案:登入後查看
統計: A(0), B(1), C(0), D(3), E(0) #3429588
統計: A(0), B(1), C(0), D(3), E(0) #3429588