題組內容
11. Nitrogen dioxide and carbon monoxide react according to the following equation:
NO2(g) + CO(g) ⇌ NO(g) + CO2(g) ΔH= −226 kJ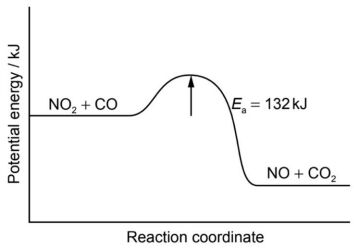
11. Nitrogen dioxide and carbon monoxide react according to the following equation:
NO2(g) + CO(g) ⇌ NO(g) + CO2(g) ΔH= −226 kJ