題組內容
三、 Suppose an n mole of ideal gas has the ratio of molar specific heats γ. It undergoes three steps to form a heat engine: A→B adiabatic expansion, B → C isothermal compression, C→ A isovolumic heating. The volume and temperature at B are (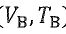 ) and the volume at A is
) and the volume at A is 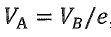 , where e is the natural logarithm(自然底數), with ln(e) = 1. The gas constant is R. Calculate
, where e is the natural logarithm(自然底數), with ln(e) = 1. The gas constant is R. Calculate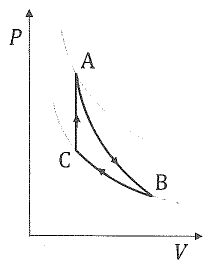
(f) the efficiency of the engine. Keep notation e in your calculations and do not substitute it by 2.71828 ....