題組內容
Question 6
One mole of a diatomic ideal gas 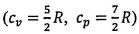 initially at 1 Pa and 298 K undergoes the following cycle:
initially at 1 Pa and 298 K undergoes the following cycle:
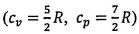 initially at 1 Pa and 298 K undergoes the following cycle:
initially at 1 Pa and 298 K undergoes the following cycle: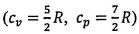 initially at 1 Pa and 298 K undergoes the following cycle:
initially at 1 Pa and 298 K undergoes the following cycle: