11. The equilibrium constant Kp = 110 for the following reaction at 400°C:
H2(g) + I2(g) ⇌2 HI(g)
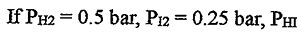 = 2 bar, which direction will this reaction go?
= 2 bar, which direction will this reaction go?
(A) The reaction goes toward products because Q<K
(B) The reaction goes toward products because Q>K
(C) The reaction goes toward reactants because Q<K
(D) The reaction goes toward reactants because Q>K
(E) The reaction does not move because the system is already at an equilibrium
答案:登入後查看
統計: A(1), B(0), C(0), D(0), E(0) #3423440
統計: A(1), B(0), C(0), D(0), E(0) #3423440