16. The Kf for the complex ion Ag(NH₃) 2+ is 1.7x107 . The Ksp for AgCl is 1.6x10-10. Calculate the molar solubility of AgCl in 1.0 M NH₃.
(A)5.2x10-2 M
(B)4.7x10-2 M
(C)2.9x10-3 M
(D)1.3x10-2 M
(E)3.5x10-2 M
答案:登入後查看
統計: A(6), B(2), C(3), D(1), E(0) #2343737
統計: A(6), B(2), C(3), D(1), E(0) #2343737
詳解 (共 1 筆)
#6366783
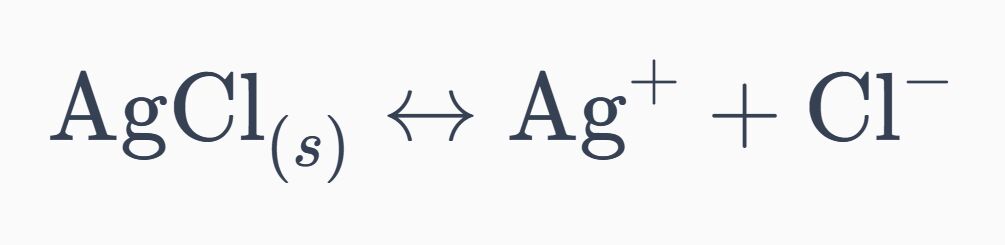
Ksp=1.6×10^−10
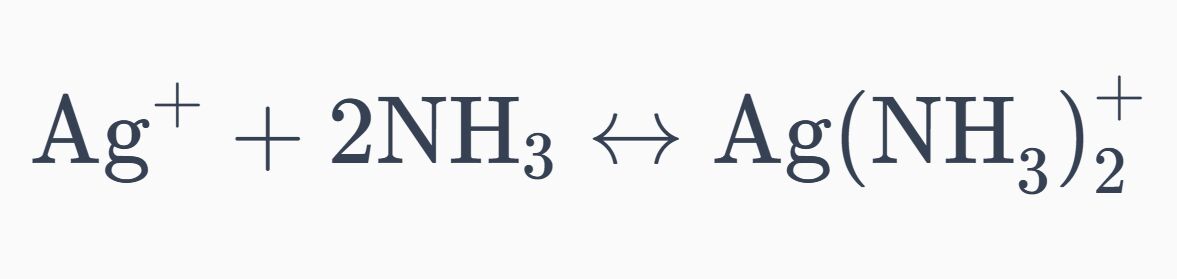
Kf=1.7×10^7
ㅤㅤ
總平衡
ㅤㅤ
假設s為AgCl的溶解度,則:
ㅤㅤ
代入總平衡常數:
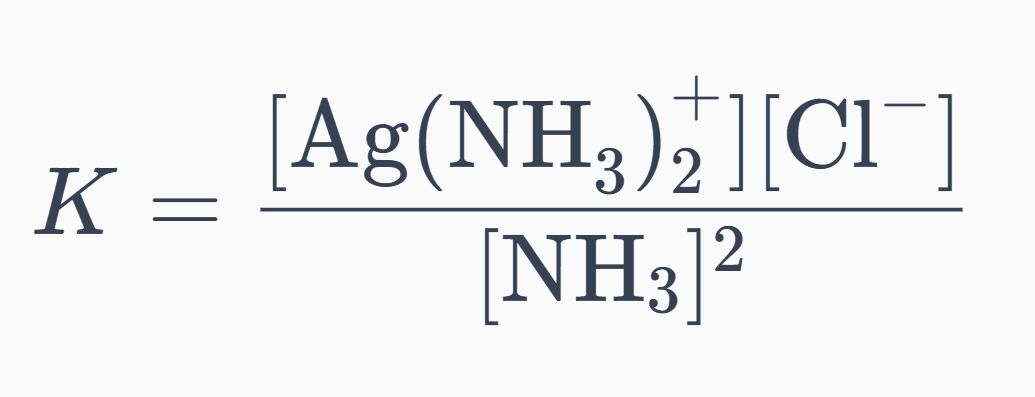
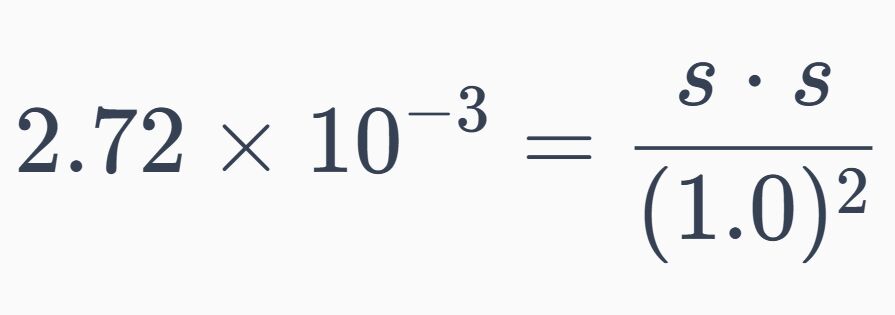
1
0