17. The complex ions of 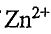 are all colorless. The most likely explanation for this is that
are all colorless. The most likely explanation for this is that
(A)  is paramagnetic.
is paramagnetic.
(B)  exhibits "d orbital" splittings in its complexes such that they absorb all wavelengths in the visible region.
exhibits "d orbital" splittings in its complexes such that they absorb all wavelengths in the visible region.
(C) because  is a
is a 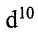 ion, it does not absorb visible light even though the "d orbital" splittings are correct for absorbing visible wavelengths.
ion, it does not absorb visible light even though the "d orbital" splittings are correct for absorbing visible wavelengths.
(D)  is not a transition metal ion.
is not a transition metal ion.
(E) because 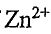 is a d8 ion, it does not absorb visible light even though the "d orbital" splitting are correct for absorbing visible wavelengths.
is a d8 ion, it does not absorb visible light even though the "d orbital" splitting are correct for absorbing visible wavelengths.
答案:登入後查看
統計: A(0), B(0), C(0), D(0), E(1) #3477622
統計: A(0), B(0), C(0), D(0), E(1) #3477622