29. Consider the following reaction:
2HF(g) ⇌H2(g) + F2(g) (K= 1.00 x 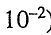 )
)
Given 1.22 mol of HF(g), 0.760 mol of H2(g), and 1.09 mol of F2(g) are mixed in a 4.00-L flask, determine the reaction quotient, Q, and the net direction to achieve equilibrium.
(A) Q=0.679; the equilibrium shifts to the right.
(B) Q=0.557; the equilibrium shifts to the left.
(C) Q=0.679; the equilibrium shifts to the left.
(D) Q=0.557; the equilibrium shifts to the right.
(E) Q=1.43; the system is at equilibrium.
答案:登入後查看
統計: 尚無統計資料
統計: 尚無統計資料