題組內容
Problem 1. A container has two gas species, where neon and argon are stored and
separated by a partition. The given conditions are shown in the image below. The molar
masses and specific heats of Ne and Ar are 20.18 kg/kmol, 39.95 kg/kmol, 0.6179
kJ/kg. ℃, and 0.3122 kJ/kg.℃, respectively. (20%; 5 pts each)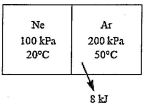
Q2. If the partition in the box is removed and a heat loss takes place during the mixing
process, how do you simplify the 1st law below to evaluate the mixture temperature
at its equilibrium state? The first law of thermodynamics for transient processes is
expressed as:
Subscripts 1 and 2 are states; subscripts i and o are inlet and outlet, respectively.