題組內容
Problem 3
A and B form an ideal solution at 30 oC, with xA = 0.4, 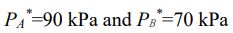
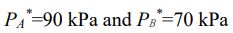
(Note: 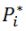 is the vapor pressure of the corresponding pure substance.
is the vapor pressure of the corresponding pure substance. 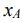 is the mole fraction of that component in the liquid)
is the mole fraction of that component in the liquid)
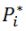 is the vapor pressure of the corresponding pure substance.
is the vapor pressure of the corresponding pure substance. 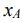 is the mole fraction of that component in the liquid)
is the mole fraction of that component in the liquid)