題組內容
1. A mixture of CO(g), H2(g), and CH3OH(g) at 500 K with 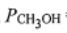 = 0.3 bar,
= 0.3 bar, 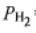 =2 bar, and
=2 bar, and 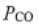 = 20 bar is passed over a catalyst.
= 20 bar is passed over a catalyst.
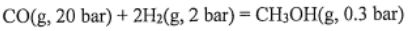
(a) The standard Gibbs energy of reaction is 21.21 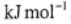 . What is the value of the Gibbs energy of reaction in
. What is the value of the Gibbs energy of reaction in 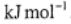 ?
?
1. A mixture of CO(g), H2(g), and CH3OH(g) at 500 K with 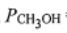 = 0.3 bar,
= 0.3 bar, 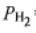 =2 bar, and
=2 bar, and 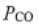 = 20 bar is passed over a catalyst.
= 20 bar is passed over a catalyst.
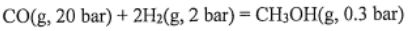
(a) The standard Gibbs energy of reaction is 21.21 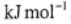 . What is the value of the Gibbs energy of reaction in
. What is the value of the Gibbs energy of reaction in 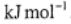 ?
?